Robotic resection of intraductal
neoplasm of the pancreas
Machado MA, Makdissi FF, Surjan RC, Abdalla RZ.
J Laparoendosc Adv Surg Tech A. 2009 19(6):771-5.

Machado MA, Makdissi FF, Surjan RC, Abdalla RZ.
J Laparoendosc Adv Surg Tech A. 2009 19(6):771-5.
Robotic Resection of Intraductal Neoplasm of the Pancreas
Marcel Autran Machado†*,MD, Fábio F. Makdissi*,MD, Rodrigo C. Surjan*,MD, Ricardo Z. Abdalla†,MD
Introduction
Laparoscopic and minimally invasive surgery development has been one of the most important advances in operative technique. Variety pancreatic lesions, such as acinar cell tumors, squamous cell carcinomas, islet cell tumors, cystic neoplasms and adenocarcinomas are most often treated by surgical resection.1
The rationale for minimally invasive pancreatic resections relies in evidences that lesser perioperative trauma in laparoscopy is advantageous when compared to open approach. This reduction results in decreased inflammatory response, preservation of the immune function and maybe even reduction of malignant recurrence.2-4
Computer-assisted surgical devices have recently been approved for general surgical use. Robotic or computer-assisted surgery is a new acquisition to the armamentarium of minimally invasive surgical techniques and remains in its infancy but may be particularly useful in advanced laparoscopic procedures such as pancreatic resections.
Robotic pancreatic resection is mentioned rarely in the English literature with only four papers found dealing with this procedure.5-8 Among them there are two case reports5,6 two papers from the same group5,7 and only two dealing directly with this procedure and with brief description of technique.5,6 There is a lack of technical description of this complex procedure. The aim of this paper is to describe the technique of a full robotic pancreatic resection in a patient with intraductal neoplasm. To our knowledge this is the first robotic pancreatic resection in Latin America and the first case of intraductal neoplasm treated by this method so far, in the English literature.
Operative Technique
A 37-year-old female with previous history of radical mastectomy for bilateral breast cancer due to BRCA2 mutation presented an acute pancreatitis episode. Radiologic investigation disclosed an intraductal pancreatic neoplasm located in the neck of the pancreas with atrophy of body and tail (Figures 1a-b). Main pancreatic duct was enlarged. Surgical decision was to perform a laparoscopic subtotal pancreatectomy using the Da Vinci robotic system. The patient is initially placed in supine position and a cushion is placed below its left flank, thus tilting the patient toward the right lateral decubitus position by approximately 30°. An orogastric tube is inserted and removed at the completion of the procedure. Using an open technique, a 11-mm trocar is placed in the supraumbilical position; through this port, robotic camera is introduced, and 4 additional ports are placed: three 8-mm and one 12-mm as displayed in Figures 1c-d.
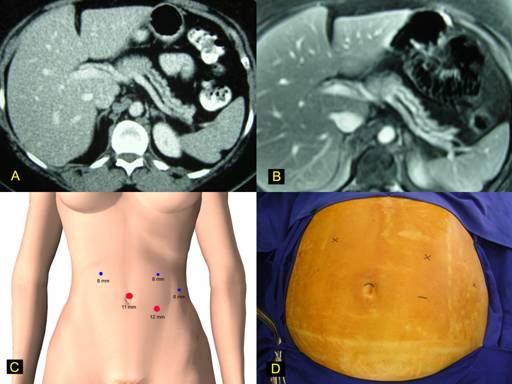
FIGURE 1. Robotic pancreatic resection.
The gastroepiploic ligament and short gastric veins are divided with harmonic scalpel (UltraCision, Ethicon Inc., Cincinatti,OH). This step permits the location and ligation of the splenic artery in the superior border of the pancreas. Posterior aspect of the pancreas at the level of the pancreatic neck is carefully dissected in order to disclose the anterior surface of portal and mesenteric veins. A robotic instrument is inserted behind pancreatic neck and the pancreas is encircled with a cardiac tape. This tape will be used during the whole procedure allowing upward traction of the pancreas (Figure 2a). Next step was to transect the pancreas using a vascular endoscopic stapler (Figure 2b). Once this accomplished, splenic vein is divided with vascular stapler and the distal pancreas is mobilized from the retroperitoneum (Figure 2c). Caution must be taken to control inferior mesenteric vein which runs through the inferior border of the pancreas. The lower pole of the spleen is mobilized through partial division of splenocolic ligament (Figure 2d).
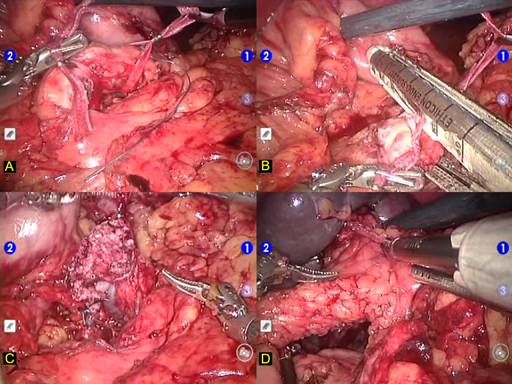
FIGURE 2. Robotic pancreatic resection surgical steps.
Dissection in completed with the mobilization of the splenic upper pole through division of splenophrenic ligament (Figure 3a). Surgical specimen containing pancreas and spleen is lifted (Figure 3b) and placed inside a plastic retrieval bag. This bag is brought through the additional 12-mm port where the spleen is morcellated without contamination of the abdominal cavity with splenic cells. Pancreatic stump is revised for hemostasis (Figure 3c). Pancreatic specimen is retrieved intact for anatomopathologic examination (Figure 3d). A round 19 F Blake abdominal drain (Ethicon,Inc, Cincinnati, Ohio) is left in place and the procedure is terminated.
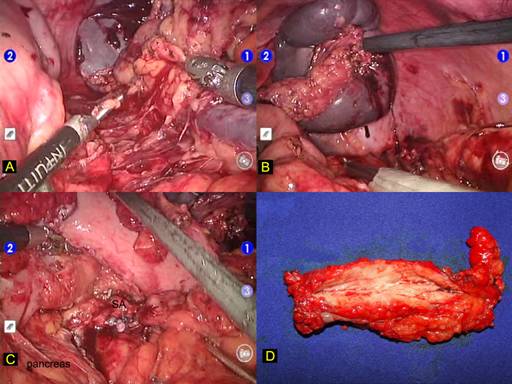
FIGURE 3. Robotic pancreatic resection technique.
Results
Operative time was 240 minutes. Blood loss was minimal and the patient did not receive transfusion. The recovery was uneventful and patient was discharged on the 4th postoperative day. There was no pancreatic leakage and drain was removed on the 7th postoperative day. Patient is well and asymptomatic six months after the procedure.
Discussion
In 1996, Salky and Edye were the first authors to advocate the use of laparoscopic surgery to treat pancreatic lesions.9 Minimally invasive surgery can reduce surgical trauma, increase safety and accelerate recovery.
Robotic surgery became reality in 1994 when a camera holder for use in laparoscopic surgery was approved by the US Food and Drug Administration (FDA) and further evolved to a voice-command system that enabled hands-off control of the laparoscope.10 The Da Vinci robotic surgical system (Intuitive Surgical, Sunnyvale, CA) made the remote control of laparoscopic instruments reality. In 1997, Cadiere and Himpens reported the first laparoscopic cholecystectomy using this robotic system.11 Three years later, in July 2000, the FDA approved the Da Vinci system for use in general surgical procedures.
The Da Vinci robotic system offers a number of advantages over traditional laparoscopy, including: a) improvements in ergonomics - the surgeon sits in a console and manipulates hand controls in a comfortable and ergonomic fashion; b) fine-motion filter eliminates natural tremors of the hands and allows motion to be scaled up to 5:1; c) significant increase in motion allowed by multi-articulated robotic instruments; d) three-dimensional visualization and e) robotic control of the camera, allowing the operating surgeon to control the visualization without movement or fatigue in a stable platform, as the camera cannot move unless when engaged by the surgeon.12
However, robotic surgical systems have its limitations. As the initial step of a new technology, it is difficult to handle and quite large. It necessitates a large operating room, imposes limitations in patient positioning and port placement must be well planned to prevent interference between operative and camera arms. Another important issue is that proprioception and tactile sensitivity is not yet available in the robotic system, making it dangerous to move instruments outside the visual field.
As experience with robotic technology increased and its applications to advanced laparoscopic procedures have become more understood, surgeons are carefully exploring the application of this innovative technology to the diseases of the pancreas. The first robotic pancreatic resection was reported by Melvin et al in 2003.5 Since then only one paper dealing directly with robotic-assisted pancreatic resection was published in the English literature.6 In this paper we report the detailed technique of a totally robotic subtotal pancreatectomy for intraductal neoplasm of the pancreas.
Although robotic technology has been applied to numerous surgical procedures, it seems that best indications of this technology are to advanced laparoscopic procedures, such as pancreatic resections. Pancreatic resections are feasible and secure, and further experience will determine the true extent of benefits provided by this ultimate technology.
The author concludes that subtotal laparoscopic pancreatic resection can safely be performed. The Da Vinci robotic system allowed for technical refinements of laparoscopic pancreatic resection. Robotic assistance improved dissection and control of major blood vessels due to 3-dimensional visualization of the operative field and instruments with wrist-type end-effectors.
References